29/07/2022
29/07/2022
 12:00 h
12:00 h
- Lecturer: Prof. Dr. Guido Clever
- University: Department of Chemistry and Chemical Biology, TU Dortmund University (Germany)
- Sponsored by:

Non-statistical Assembly of Functional Coordination Cages
Advanced self-assembly strategies enable the targeted synthesis of supramolecular systems and materials with increasing structural and functional complexity. We use bis-monodentate ligands, reacting with transition metal cations such as Pd(II) to coordination compounds showing a broad range of topologies from small Pd2L4 cages, their interpenetrated dimers, rings of various size up to large Pd24L48 spheres.1 Further, we introduce stimuli-responsive behaviour triggered by small molecules or light leading to, e.g., the modulation of guest affinity2 or complete structural reorganization.3
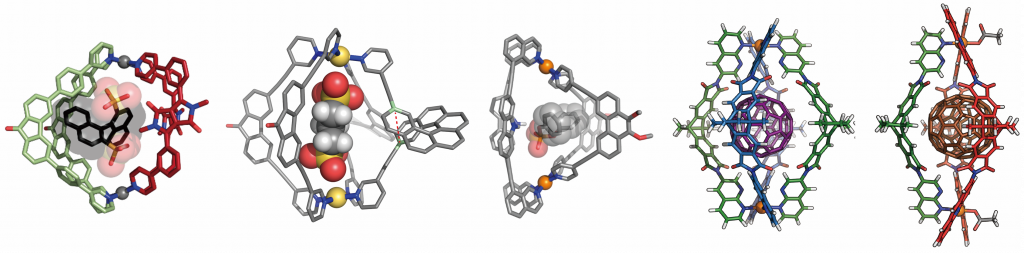
In order to combine different functionalities in the same metallosupramolecular assembly, we develop non-statistical assembly strategies to obtain heteroleptic cages with defined structures and composition. The “shape complementary assembly” (SCA) approach, leading to integrative self-sorting of carefully designed building blocks with matching dimensions, and the “coordination sphere engineering” (CSE) method, allowing control over steric and electronic ligand interplay right at the metal center, will be presented.4 Examples of functional heteroleptic cages include hosts that show guest-modulated circularly polarized luminescence (CPL) based on chirality transfer (Figure 1, left two structures)5 and phosphate ester binding through endohedral hydrogen bond donors and π-surfaces (Figure 1 middle). Also, fullerene encapsulation was achived,6 followed by confinement-controlled reactivity, e.g. selective Diels-Alder addition and long-term C60 radical anion stabilization7 (Figure 1, right two structures).
Figure 1. Examples of heteroleptic coordination cage structures with guest-modulated chiroptical emission (left), phosphate binding (middle) and fullerene uptake (right).
References:
1 S. Pullen, G. H. Clever, Acc. Chem. Res. 2018, 51, 3052.
2 H. Lee, J. Tessarolo, D. Langbehn, A. Baksi, R. Herges, G. H. Clever, J. Am. Chem. Soc. 2022, 144, 3099.
3 M. Han, Y. Luo, B. Damaschke, L. Gómez, X. Ribas, A. Jose, P. Peretzki, M. Seibt, G. H. Clever, Angew. Chem. Int. Ed. 2016, 55, 445.
4 S. Pullen, J. Tessarolo, G. H. Clever, Chem. Sci. 2021, 12, 7269.
5 K. Wu, J. Tessarolo, A. Baksi, G. H. Clever, Angew. Chem. Int. Ed. 2022, DOI: 10.1002/anie.202205725.
6 B. Chen, J. J. Holstein, A. Platzek, L. Schneider, K. Wu, G. H. Clever, Chem. Sci. 2022, 13, 1829.
7 S. Hasegawa, S. L. Meichsner, J. J. Holstein, A. Baksi, M. Kasanmascheff, G. H. Clever, J. Am. Chem. Soc. 2021, 143, 9718.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements