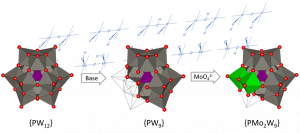
The ability of group VI cluster oxides to act as electron sponges is widely known[1] hence the tantalizing prospect of potentially using them as photocatalysts. In one of these attempts dating back to the beginning of this century, the classic Keggin structure [H3PW12O40] was deconstructed to incorporate two Mo(VI) addenda (Figure 1). This allowed for the subsequent single-electron reduction of this mixed metal structure to have a well-defined electron carrier in this new {Mo2} moiety, as Mo(VI) is a better oxidant than W(VI).
This mono-reduced salt is formulated as [N(C4H9)4]4[H4PW9Mo2O39] and NMR experiments provided a measurement of the kinetics of electron hopping between metal sites.
There was however one major obstacle that prevented the publication of these results: no crystal structure!
By deploying computational tools and some chemical intuition, light is shed on the isomer energetics of the plausible lacunary structures of the [H4PW9Mo2O39]4- anion and the electron transfer phenomena occurring in the NMR timescale. [2]
Figure 1 – Design route for mixed addenda tungsto-molybdates.
[1] M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, 1983.
[2] N. A. G. Bandeira, H. Liu, M. J. Calhorda, J. Chem. Phys. 2021, 154, 124301. https://dx.doi.org/10.1063/5.0039092
This event will be recorded and uploaded on ICIQ’s YouTube Channel.
If you are interested in attending this talk, please, register here!