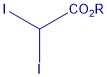
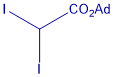
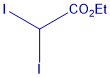
Metal-free cyclopropanation reagent
Technology Status: TRL 3 (tested in laboratory) PATENT APPLICATION FILLED
Sectors of Application: Medicinal chemistry, R&D chemistry, synthetic chemistry, drug discovery and development, fine chemistry, fragrances
Introduction
The research group of Marcos Garcia Suero from the ICIQ has developed a shelf-stable solid reagent for metal-free photochemical method for the preparation of cyclopropanes from substrates incorporating a C-C double bond conjugated to an aromatic or heteroaromatic group. The cyclopropanation advantageously takes place without photosensitizer or metal catalyst. Over the last few decades, one of the main challenges in the synthesis of cyclopropane rings has been to develop robust and general alkene cyclopropanations that avoid the use of explosive, highly toxic, pyrophoric or water-sensitive carbene transfer reagents (diazo reagents and organometallic carbenoids). However, although important advances have been made in this direction, a major limitation remaining is the broad tolerance to functional groups and consequently, the cyclopropanation of complex molecules.
Description of solution
This innovation reports the first general photocatalyst-free cyclopropanation for substances with double bonds between carbon conjugates with an aromatic/heteroaromatic groups that uses underexploited gem-diiodomethyl carbonyl compounds as cyclopropanating reagents (Figure X) and a simple compact fluorescence lamp as a visible-light source. This process give rise to valuable cyclopropylcarboxylates that could be easily diversified. Cyclopropanation of styrene-like substrates can be carried out under visible light irradiation with no need of a sensitizer and using shelf-stable reagents.
This technology has an excellent functional group tolerance and has a high chemoselectivity that has enabled access to cyclopropyl carboxylates. The excellent functional group tolerance and chemoselectivity observed towards the styrene double bond are in sharp contrast to current catalytic methods that cyclopropanate styrenes involving electrophilic metal-carbenes from diazoacetates. One of the more important advantages is that the cyclopropanation reagent consist on a shelf-stable solid which makes it useful as a reagent. The synthesis of the reagent is easily scalable. Due to the mild conditions and metal-free cyclopropanation process, the process can be considered a green process.
Advantages and unique selling points
- Robust technology: The technology has been proved on 52 different substrates, including heterocycles and biomolecules, with up to 93% yield for the cyclopropanation product.
- Excellent functional group tolerance: This technology has an excellent functional group tolerance and has a high chemoselectivity that has enabled access to cyclopropyl carboxylates.
- Late-stage functionalization: As an additional advantage, the method tolerates very well the presence of functional groups on the reaction substrate, which makes it suitable for late-stage functionalization.
- Shelf-stable solid: The cyclopropanation reagent consist on a shelf-stable solid which makes it useful as a reagent.
Status of Technology
Laboratory scale tests have been performed at the Marcos Garcia Suero’s group.
Type of partnership sought
An industrial partner is sought for the commercialization and distribution of the cyclopropanation reagent through exclusive or non-exclusive license agreements.
IP Situation
An European patent application has been filed:
1-Title of the patent: Cyclopropanation method and reagent
-Application number: EP19382720
-Filing date: 22/08/2019
-Priority date: 22/08/2019
References