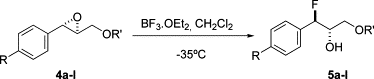
Ring-opening hydrofluorination of enantiomerically pure (2S,3S)-3-arylglycidyl ethers (aryl = phenyl, 4-trifluoromethylphenyl) by boron trifluoride-diethyl ether under mild conditions provides ß-fluoro alcohols in good yield in a stereospecific manner with complete regiocontrol.