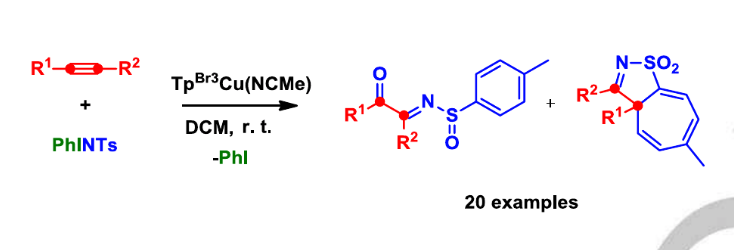
A novel transformation is reported for the reaction of terminal or internal alkynes with the nitrene precursor PhI=NTs (Ts = p-toluenesulfonyl) in the presence of catalytic amounts of TpBr3Cu(NCMe) (TpBr3 = hydrotris(3,4,5-tribromo-pyrazolylborate). Two products containing an imine functionality have been isolated from the reaction mixtures, identified as sulfinamides and iso-thiazoles. The former correspond to the formal reduction of the sulfone group into sulfoxide, whereas the latter involves the insertion of an alkyne carbon atom into the aromatic ring of the N-tosyl moiety.