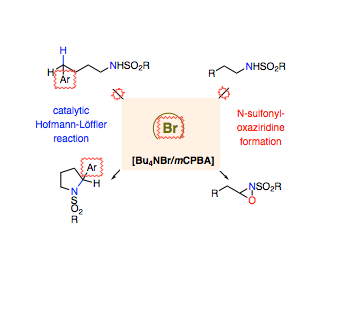
The potential of homogeneous oxidation catalysis employing bromine has remained largely unexplored. We herein show that the combination of a tetraalkylammonium bromide and meta‐chloroperbenzoic acid offers a unique catalyst system for the convenient and selective oxidation of saturated C(sp3)−H bonds upon photochemical initiation with day light. This approach enables remote, intramolecular, position‐selective C−H amination as demonstrated for 20 different examples. For the first time, an N‐halogenated intermediate was isolated as the active catalyst state in a catalytic Hofmann–Löffler reaction. In addition, an expeditious one‐pot synthesis of N‐sulfonyl oxaziridines from N‐sulfonamides was developed and exemplified for 15 transformations. These pioneering examples provide a change in paradigm for molecular catalysis with bromine.