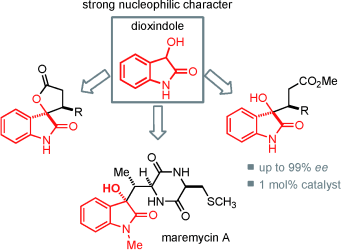
Understanding the nucleophilicity of dioxindole under different reaction conditions is key to a direct and easy access to valuable spiro oxindole γ?butyrolactones and 3-substituted 3-hydroxyoxindole derivatives in excellent yields and enantioselectivities (see scheme). The preparation of maremycin?A serves as an example for the potential usefulness of this previously unexplored reactivity in natural product synthesis.