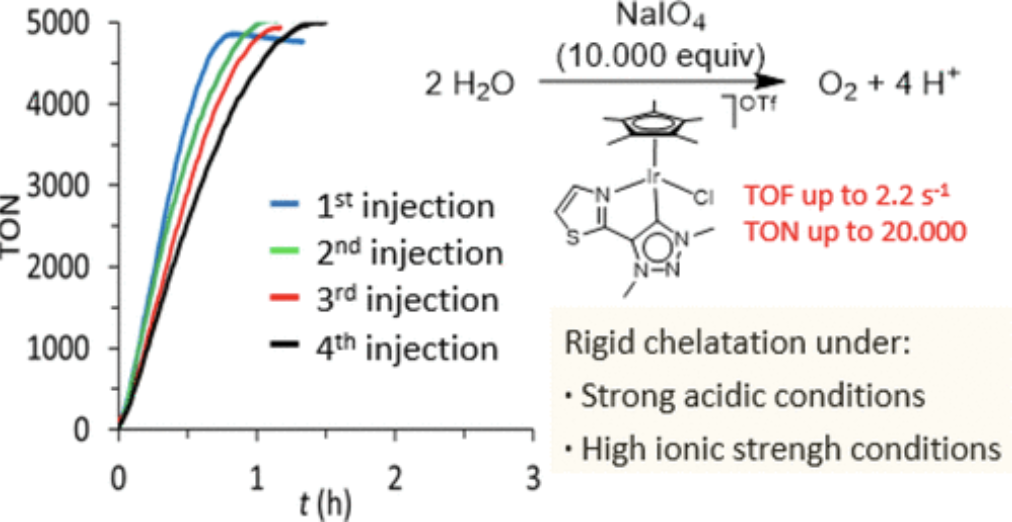
We report the effect of replacing the pyridine group in the chelating trz Ir–water oxidation catalysts by a benzoxazole and a thiazole moiety. We have also evaluated if the presence of bidentate ligands is crucial for high activities and to avoid the decomposition into undesired heterogeneous layers. The catalytic performance of these benzoxazole/thiazole–triazolidene Ir-complexes in water oxidation was studied at variable pH using either CAN (pH = 1) or NaIO4 (pH = 5.6 and 7). Electrocatalytic experiments indicated that while CAN-mediated water oxidation led to catalyst heterogeneization irrespective of the triazolylidene substituent, periodate as sacrificial oxidant preserved a homogeneously active species. Repetitive additions of sacrificial oxidant indicates higher integrity of the Ir-complex with a thiazole-substituted triazolylidene compared to ligands featuring a benzoxazole as chelating donor or no chelating group at all. Rigid chelation of the thiazole group was also established from stability measurements under highly acidic, oxidizing, and high ionic strength conditions.