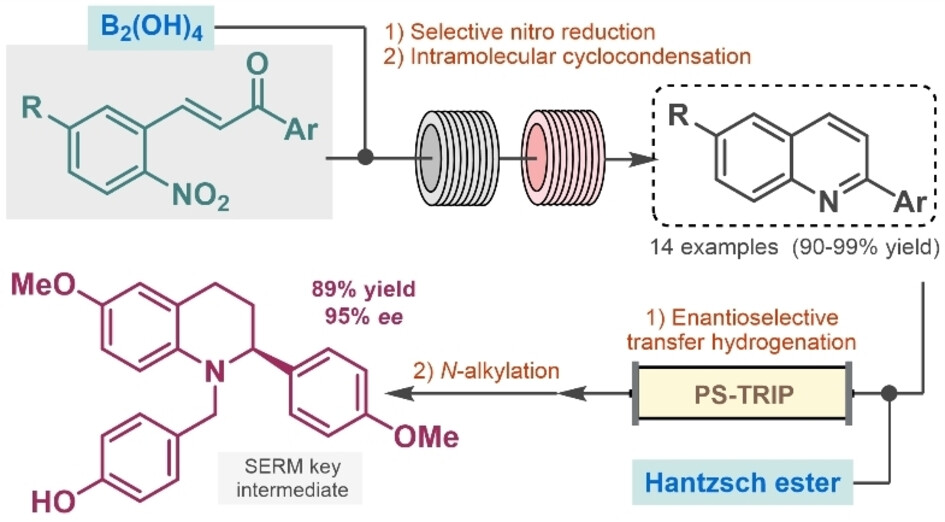
An asymmetric enantioselective flow process is reported for the formal synthesis of a 1,2,3,4-tetrahydroquinoline selective estrogen receptor modulator. Starting from an easily available 2-nitrochalcone, the first part of the process comprised a telescoped nitro reduction/intramolecular cyclocondensation sequence using diboronic acid as a simple reductant. Subsequent enantioselective transfer hydrogenation in the presence of an immobilized phosphoric acid organocatalyst followed by telescoped N-alkylation furnished the targeted chiral intermediate. The approach ensures flexibility regarding the scale of the synthesis, whilst minimizing the need for intermediate purifications and ensuring environmentally benign metal-free conditions.