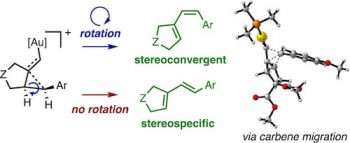
We identify the factors that rule the selectivity in single-cleavage skeletal rearrangements promoted by gold(I) catalysts. We find that stereoconvergence is enabled by a rotational equilibrium when electron-rich substituents are used. The anomalous Z-selective skeletal rearrangement is found to be due to electronic factors, whereas endo-selectivity depends on both steric and electronic factors.