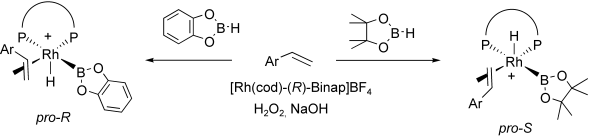This study attempts to rationalise the unpredictable performance of transition metal catalysed asymmetric hydroboration of vinylarenes on varying the precursor of the catalyst from cationic to neutral species, [M(cod)(L-L)]BF4, [M(n-Cl)(cod)]2/(L-L), the metal (M=Rh and Ir), and the hydroborating reagent (catecholborane, pinacolborane). The approaches are based on the agreement between experimental data provided by (R)-Binap and (R)-Quinap modified catalytic systems and computational data evidenced by DFT calculations and QM/MM strategies. Unprecedentedly high enantiomeric excesses in the hydroboration/oxidation of vinylarenes with both electron-withdrawing substituents ((R)-(+)-1-p-F-phenylethanol, ee up to 92 %) and electron-releasing substituents ((R)-(+)-1-p-MeO-phenylethanol, ee up to 98 %), can be attributed to a rhodium halide key intermediate.