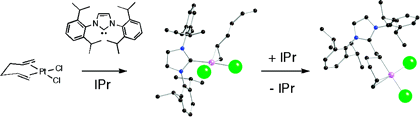
The insertion of a N-heterocyclic carbene (NHC) into a coordinated olefin moiety is observed for a Pt-based system. Important intermediates along the reaction pathway have been isolated and fully characterized. Computational work supports a proposed mechanistic pathway involving external attack at the olefin by a NHC ligand rather than an intramolecular migratory insertion route.