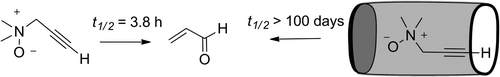Thermally and in nonprotic media N,N-dimethyl-2-propyn-1-amine N-oxide undergoes two consecutive sigmatropic rearrangements affording propenal. Two molecular containers capable of the quantitative inclusion/encapsulation of the N-oxide are described. The N-oxide becomes kinetically stabilized when included in the containers. The relationship between the observed kinetic stabilization of the N-oxide and the thermodynamic and kinetic stability of its inclusion complexes is explained and modeled.