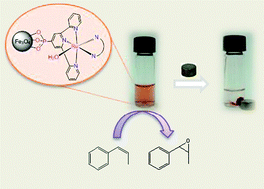
Two new Ru-aqua complexes containing a phosphonated trpy ligand with the general formula [Ru(trpy-P)(B)(H2O)]2+ (trpy-P is diethyl [2,2′:6′,2′′-terpyridin]-4′-ylphosphonate (1); B = bpm (5a) is 2,2′-bipyrimidine; (or) B = azpy (cis- and trans-5b) is 2-phenylazopyridine) are reported. These complexes and their synthetic intermediates have been characterized by the usual analytic techniques and by spectroscopic and electrochemical methods. X-ray structures of the cis and trans Ru-Cl complexes that are synthetic intermediates to the corresponding Ru-aqua complexes have also been obtained. The phosphonated complexes 4a and trans-4b have been anchored onto MNPs of Fe3O4 (6.2 ± 2 nm), via covalent bonds to generate 6a and trans-6b, respectively. These new materials have been characterized by UV-vis, IR, TEM and CV. The Ru-aqua complexes were generated in situ by dissolving the Ru-Cl complexes in water. The Ru-aqua complexes 5a and trans-5b are excellent catalysts for the epoxidation of olefins and are stereoselective for cis-alkenes such as cis-β-methylstyrene and cis-stilbene. Remarkably, the analog supported onto MNPs, 7a, displays practically the same behavior as its homogeneous counterpart. Multiple recycling experiments for the epoxidation of cis-β-methylstyrene involving magnetic decantation of the catalyst were carried out with 7a without significant loss of activity.