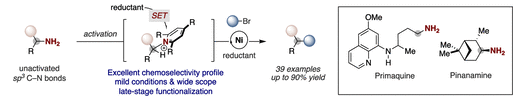
A Ni-catalyzed reductive deaminative arylation at unactivated sp3 carbon centers is described. This operationally simple and user-friendly protocol exhibits excellent chemoselectivity profile and broad substrate scope, thus complementing existing metal-catalyzed cross-coupling reactions to forge sp3 C–C linkages. These virtues have been assessed in the context of late-stage functionalization, hence providing a strategic advantage to reliably generate structure diversity with amine-containing drugs.