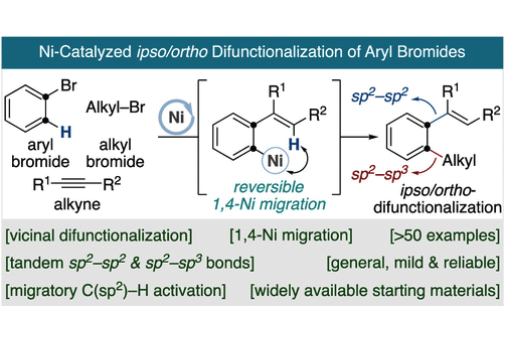
Polysubstituted arenes are ubiquitous structures in a myriad of medicinal agents and complex molecules. Herein, we report a new catalytic blueprint that merges the modularity of nickel catalysis for bond formation with the ability to enable a rather elusive 1,4-hydride shift at arene sp2 C–H sites, thus allowing access to ipso/ortho-difunctionalized arenes from readily available aryl halides under mild conditions and exquisite selectivity profile.