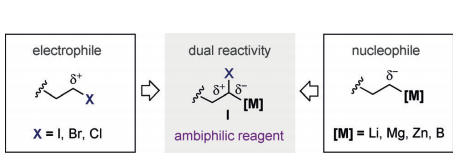
A nickel-catalyzed reductive arylation of ambiphilic α-bromoalkyl boronic esters with aryl halides is described. This platform provides an unrecognized opportunity to promote the catalytic umpolung reactivity of ambiphilic reagents with aryl halides, thus unlocking a new cross-coupling strategy that complements existing methods for the preparation of densely functionalized alkyl-substituted organometallic reagents from simple and readily accessible precursors.