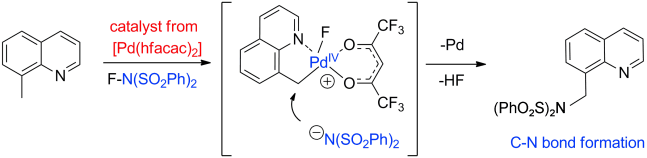
A new palladium-catalyzed intermolecular sequence consisting of the C=H activation and amidation of methyl groups relies on N-fluorobis(phenylsulfonyl)imide (NFSI) as both the oxidant and the nitrogen source. The reaction provides the corresponding arylamines as bissulfonimides along with HF as the only by-product. Both experimental and computational results suggest the involvement of a monomeric palladium(IV) intermediate.