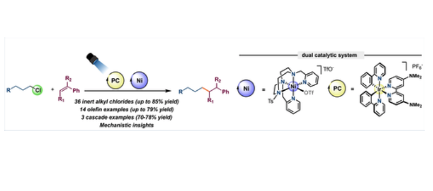
The photoredox activation of inert chloroalkanes for the cross-coupling reaction with olefins, with a broad functional group tolerance under mild conditions is presented. By combining UV/Vis spectroscopy, EPR, radical clock and deuterium-labelling experiments, [Ni(Py2Tstacn)]+ is proposed to be catalytically competent to activate the Csp3−Cl bond, forming a free radical, which reacts with the alkene.