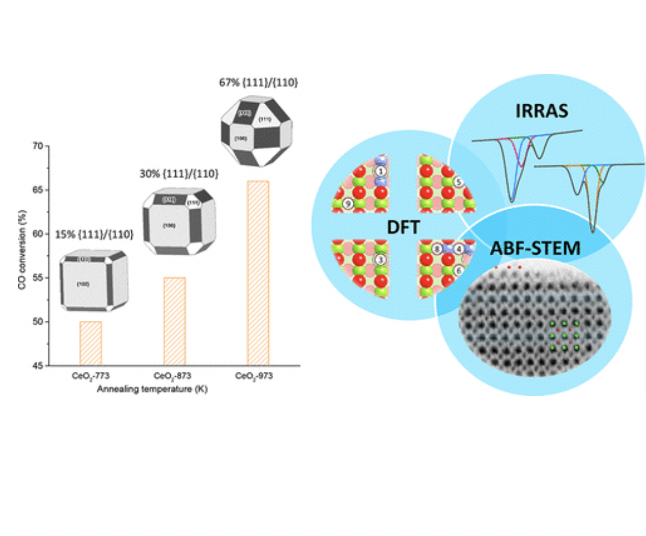
Polar surfaces of solid oxides are intrinsically unstable and tend to reconstruct due to the diverging electrostatic energy, and thus often exhibit unique physical and chemical properties. However, a quantitative description on the restructuring mechanism of these polar surfaces remains challenging. Here, we provide an atomic-level picture of the refaceting process that governs the surface polarity compensation of cubic ceria nanoparticles, based on the accurate reference data acquired from the well-defined model systems. The combined results from advanced infrared spectroscopy, atomic-resolved transmission electron microscopy and density functional theory calculations identify a two-step scenario, where an initial O-terminated (2×2) reconstruction is followed by a severe refaceting via massive mass transport at elevated temperatures to yield {111}-dominated nanopyramids. This significant surface restructuring promotes the redox properties of ceria nanocubes, which account for the enhanced catalytic activity for CO oxidation.