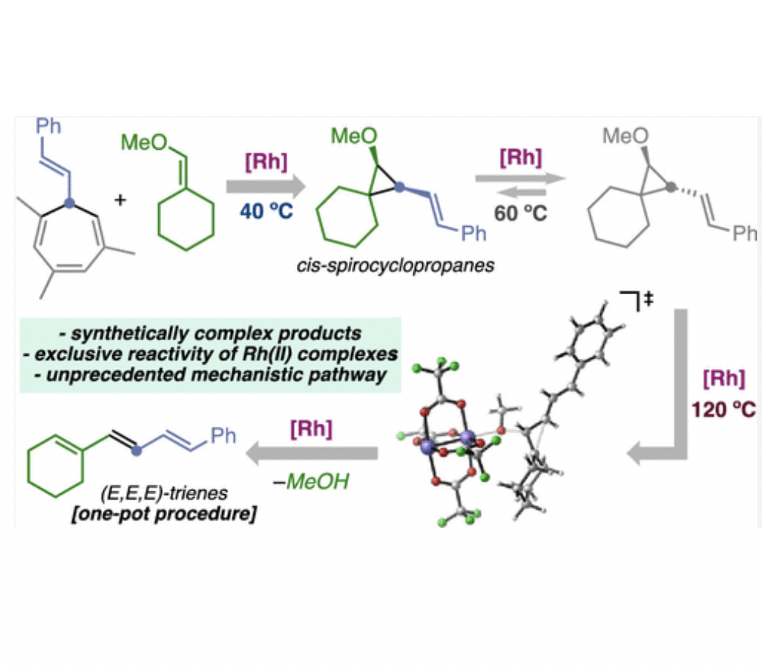
The rhodium(II)-catalyzed assembly of densely-substituted cis-configured cyclopropyl ethers by decarbenation of cycloheptatrienes is reported. At higher temperature, these non-acceptor cyclopropanes open to give all-E trienes under the same catalytic system, in a one-pot procedure. Experimental and computational studies show that, for the formation of all-E trienes, the cyclopropanes undergo first a Rh(II)-catalyzed cis- to trans-isomerization, followed by C–C bond cleavage.