Abstract
Non-covalent interactions play a crucial role in all manner of chemical and biological processes. Relatively recently, the incorporation of non-covalent interactions into the design of small molecule catalysts has revolutionised the field of enantioselective catalysis in the field now known as organocatalysis. Our research is centred around applying non-covalent catalysis to tackle outstanding selectivity challenges in synthetic chemistry.
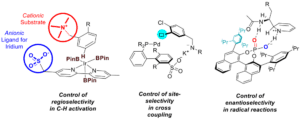
The use of non-covalent interactions to direct reactive transition metals in order to tackle issues of regioselectivity or site-selectivity in synthetic chemistry is a relatively underexplored strategy, despite obvious potential. Advances in metal-catalysed C-H functionalisation have provided many methods to functionalise arenes but arguably the outstanding challenge in this area is control of selectivity. The first part of the talk will describe the development of a multifunctional, anionic bipyridine ligand able to control regioselectivity in the iridium-catalysed C-H borylation of arenes. This single ligand is able to operate in two modes – ion pairing mode and hydrogen bonding mode, in order to direct C-H borylation the meta position of arenes with very high levels of regioselectivity on a wide range of substrates. We have subsequently applied this basic strategy to the site selective cross coupling of molecules bearing multiple chlorides. This was achieve by repurposing sulfonylated phosphine ligands which are more typically used to impart water solubility to transition metal complexes.
In the area of enantioselective catalysis, non-covalent catalysis has most commonly been applied to two-electron processes. The recent surge of development of single electron processes, largely due to the popularisation of photoredox catalysis, has highlighted the continuing challenge of controlling enantioselectivity in radical reactions. The second part of the talk will describe our studies in applying non-covalent catalysis to the control of enantioselectivity in radical processes, specifically the Minisci-type addition of prochiral radicals to pyridines and quinolines.