Abstract
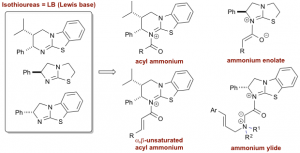
Lewis base-mediated reactions encompass a plethora of molecular transformations. Within this area, we have developed a range of catalytic (asymmetric) processes mediated by a range of Lewis bases.1 This presentation will describe recent developments in the use of isothioureas to promote a number of selective transformations that involve the generation of either an acyl ammonium,2 ammonium enolate,3 a,b-unsaturated acyl ammonium4 or ylide intermediate5 (Figure 1). The application of these methodologies to the (enantioselective) synthesis of a variety of heterocyclic products, as well as mechanistic insights into these processes will be discussed.
Figure 1: Isothioureas and intermediates in enantioselective Lewis base catalysis