Abstract
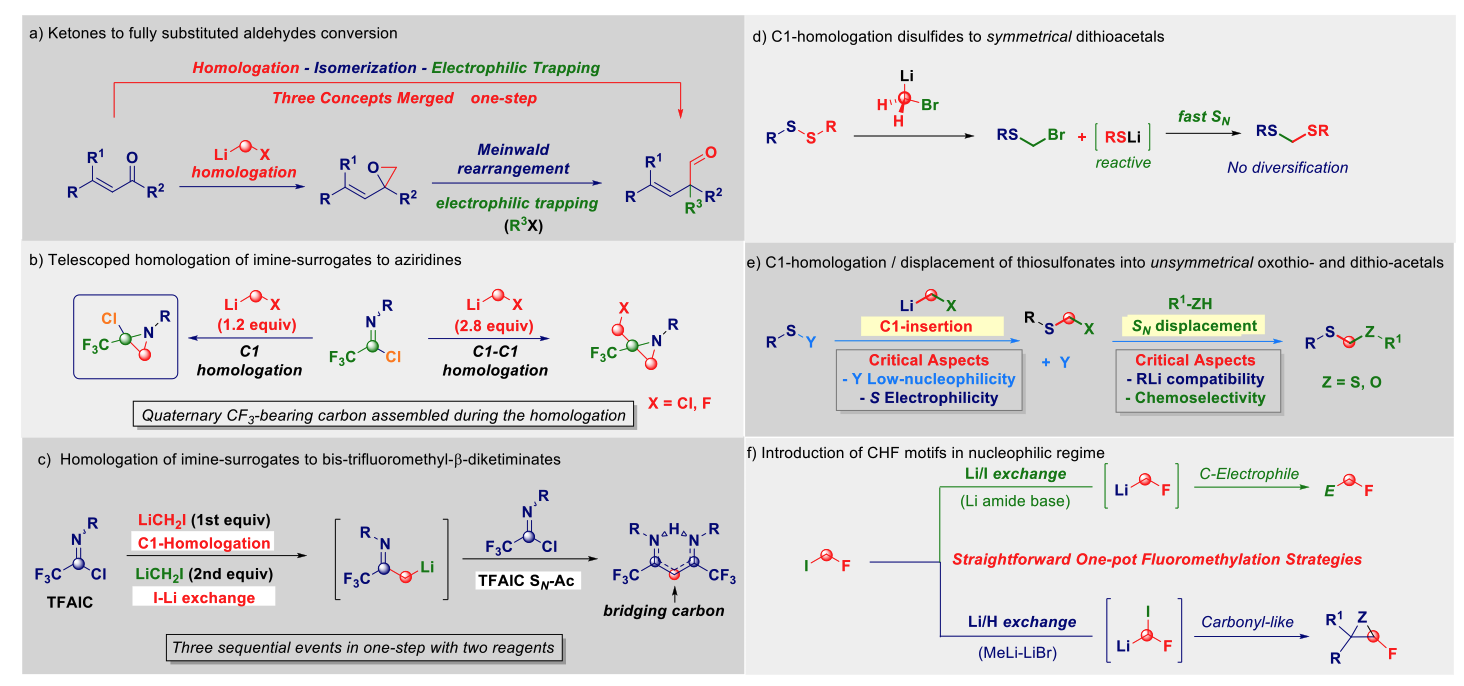
The direct transfer of a reactive nucleophilic CH2X unit into an existing linkage enables the formal introduction of the moiety with the precisely defined degree of functionalization.1 Upon the fine tuning of the reaction conditions governing the transformation, the initial homologation event can serve as the manifold for triggering unusual rearrangement sequences leading to complex architectures through a unique synthetic operation. The direct – full chemoselective – conversion of a ketone into the homologated all-carbon quaternary aldehyde (via a)2, the telescoped homologation of imine-surrogates to quaternary aziridines (via b)3 and bis-trifluoromethyl-β-diketiminates (via c) will illustrate these unprecedented concepts. Additionally, the homologation of disulfides and thiosulfonates will furnish symmetrical (via d) and unsymmetrical oxothio- and dithio-acetals (via e). The one-step mono-fluoromethylation of carbon electrophiles with extremely labile fluoromethyllithium reagents will provide a novel entry to valuable fluorinated building-blocks without the needing of using protecting elements for fluoro-containing carbanions (via f).4 Finally, the development of homologation strategies not relying on the use of external C1-sources will be discussed.5
References
(1) (a) Castoldi, L.; Monticelli, S.; Senatore, R.; Ielo, L.; Pace, V. Chem. Commun. 2018, 54, 6692-6704. (b) Senatore, R.; Castoldi, L.; Ielo, L.; Holzer, W.; Pace, V. Org. Lett. 2018, 20, 2685-2688. Homologation Reactions. Reagents, Applications and Mechanisms. (Pace, V. Ed.) Wiley-VCH, Weinheim 2023 (ISBN: 978-3-527-34815-2).
(2) Pace, V.; Castoldi, L.; Mazzeo, E.; Rui, M.; Langer, T.; Holzer, W. Angew. Chem. Int. Ed. 2017, 56, 12677-12682.
(3) (a) Ielo, L.; Touqeer, S.; Roller, A.; Langer, T.; Holzer, W.; Pace, V. Angew. Chem. Int. Ed. 2019, 58, 2479-2484. (b) Ielo, L.; Castoldi, L.; Touqeer, S.; Lombino, J.; Roller, A.; Prandi, C.; Holzer, W.; Pace, V. Angew. Chem. Int. Ed. 2020, 59, 20852-20857. (c) Senatore, R.; Malik, M.; Langer, T.; Holzer, W.; Pace, V. Angew. Chem. Int. Ed. 2021, 60, 24854-24858.
(4) (a) Parisi, G.; Colella, M.; Monticelli, S.; Romanazzi, G.; Holzer, W.; Langer, T.; Degennaro, L.; Pace, V.; Luisi, R. J. Am. Chem. Soc. 2017, 139, 13648-13651. (b) Monticelli, S.; Colella, M.; Pillari, V.; Tota, A.; Langer, T.; Holzer, W.; Degennaro, L.; Luisi, R.; Pace, V. Org. Lett. 2019, 21, 584-588. (c) Senatore, R.; Malik, M.; Spreitzer, M.; Holzer, W.; Pace, V. Org. Lett. 2020, 22, 1345-1349. (d) For sequential nucleophilic additions – deoxygenations, see: Miele, M.; Citarella, A.; Langer, T.; Urban, E.; Zehl, M.; Holzer, W.; Ielo, L.; Pace, V. Org. Lett. 2020, 22, 7629-7634.
(5) Malik, M.; Senatore, R.; Langer, T.; Holzer, W.; Pace, V. Chem. Sci. 2023, 14, 10140-10146.
